Introduction of Colour and Optical Effects In Gemstones
Welcome to our journey into “Colour and Optical Effects In Gemstones.” We’re diving into the magic of gemstone hues and optical mysteries. From understanding why gemstones have colours to exploring fascinating theories, let’s uncover the beauty and science behind these precious stones.
Table of Contents
What is Colour
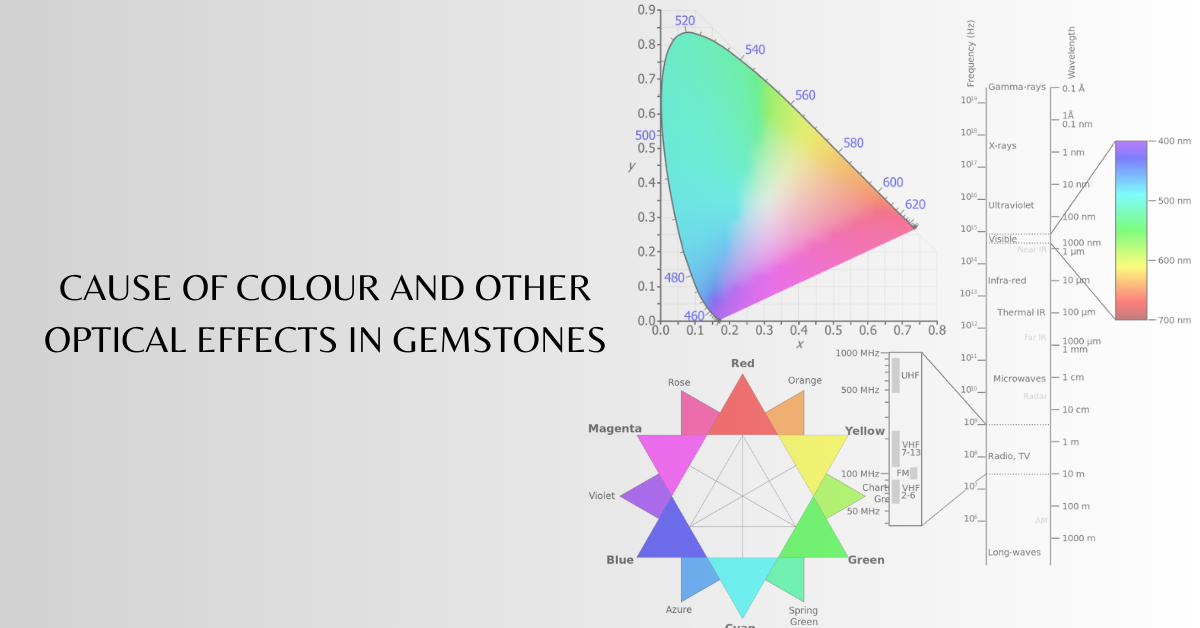
We perceive colour due to selective absorption, transmission or reflection of wavelengths of light. Light is an electromagnetic radiation. When the wavelength is within the visible spectrum (approximate from 400 nm to 700 nm), it is known as “visible light”.
These colours can be produced by visible light of a single wavelength and are called pure spectral or monochromatic colours. When white light falls on an object, say blue turquoise, the turquoise absorbs all wavelengths other than the blue, which is reflected back. This reflected light fans on the retina of our eye and the signals are transmitted to the brain, The brain interprets this as the blue colour of turquoise, Similarly, in case of a ruby, when white light passes through the crystal, green and violet wavelengths are absorbed and the remaining light, which is mostly red with some yellow and orange gives the red colour in a ruby, The human eye can distinguish about 10 million different colours. It is essential to observe the stone in a neutral white light to judge the real colour, If the incident light is rich in blue or red wavelengths the actual colour may not be judged.
| Colour | Wavelength |
|---|---|
| Violet | 390-450 nm |
| Blue | 450-490 nm |
| Green | 490-560 nm |
| Yellow | 560-590 nm |
| Orange | 590-635 nm |
| Red | 635-700 nm |
The CIE system is a universally accepted standard for colour specification and measurement. The CIE system can be understood by the “Chromaticity diagram” as illustrated. The pure spectral colours are depicted along the horseshoe shaped outer line. Saturated colours are seen along this outer line of the horseshoe, While the central point is white. The line connecting 400 and 700 nm represents various shades of purple and magenta.
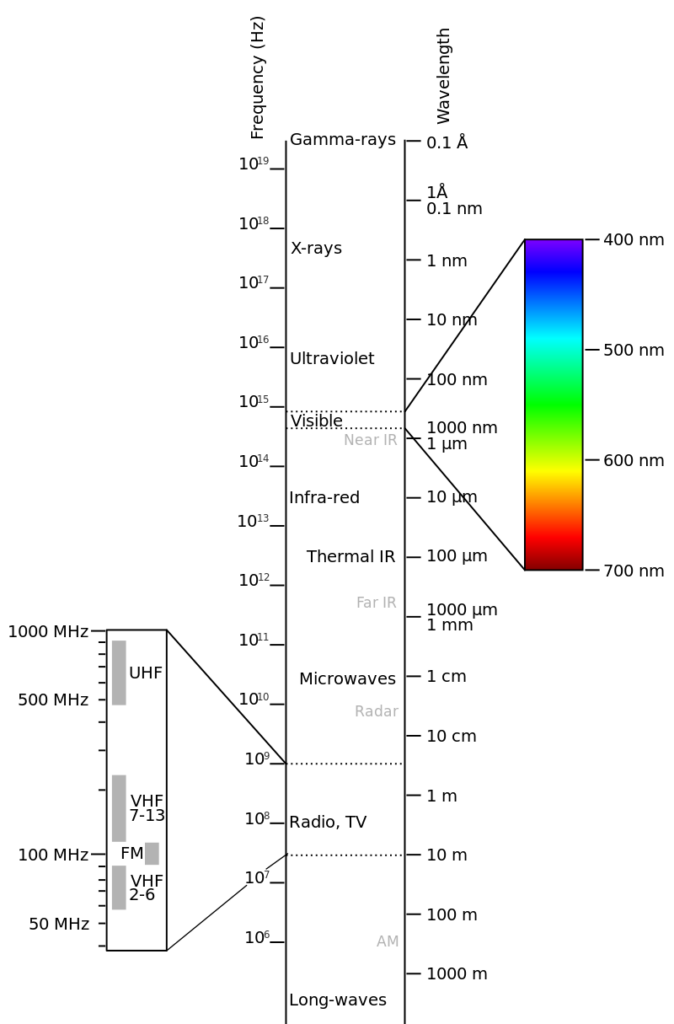
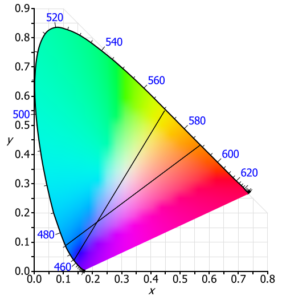
A mixture of colours on the opposite side of the horseshoe e.g. yellow and blue, in varying proportion will produce the colours shown in between, including white. Similarly, orange and blue-green in the correct proportion can produce white light. If from this white light, we remove the orange component, then blue-green remains.
Complementary pair is a set of colours which, when mixed in correct proportion. produce a neutral colour (white). Colours on opposite Sides of any line passing through the white spot and intersecting at the periphery of the horseshoe are called complementary colours.
However. when we mix complementary colour pigments, we do not obtain white but black or grey. As discussed above, green pigment appears green because it absorbs all light except green. If we mix this with a violet pigment, which absorbs all light except violet, we get black colour or dark grey colour.
The CIE system also includes a set of defined standard illuminates representing an incandescent lamp (2856K), noon sunlight (4874K), average daylight (6774K) and D65 daylight (6500 K).
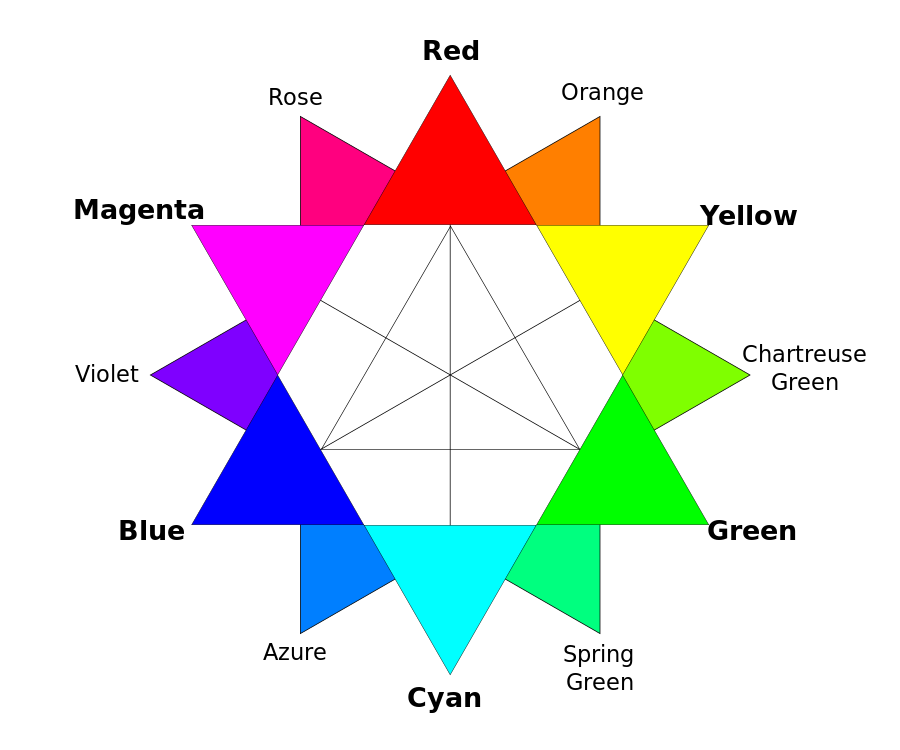
The Colour Wheel
A colour Wheel helps us to understand the relationships between colours. Primary colours yellow, blue and red form an equilateral triangle. Green, orange and purple are secondary colours, which are obtained by mixing the primary colours in same ratio. When a primary and a secondary colour is mixed, it gives rise to a tertiary colour. Col ours opposite one another are complementary. In case of aquamarine the blue-green colour is seen because of the combination of blue and yellow primary colour. The red of ruby is a combination of red primary colour and purple secondary colour.
Cause of Colour in Gemstones
Rubies and emeralds would be colourless, but for the tiny percentage of chromium oxide found in these gemstones. Similarly, tourmalines display a wide range of colours because of various chemical impurities like iron, manganese, copper or chromium. These impurities are called ‘Colouring agents’ or “chromophores” Transition elements are the most common cause of colour found in gemstones. The transition elements have the ability to absorb energy from the light rays that pass through the crystal, giving rise to the colour in the gemstone. The colour causing transition elements are Vanadium (V), Chromium (Cr), Iron (Fe), Nickel (Ni), Manganese (Mn), Copper (Cu) and Cobalt (Co). The colour we see is due to partial absorption and partial transmission of wavelengths when white light falls on a gemstone.
When white light falls on the stone, the electrons absorb some wavelengths and get excited to a higher energy level known as excited state. The unabsorbed wavelengths pass through the material giving its colour, The colour we see is complementary to the absorbed light or colours, For example, in case of ruby, white light passes through the crystal, green and violet wavelengths are absorbed to excite the electrons from the ground level to the excited level; the remaining light is mostly red with some yellow and orange which are Visible as the components of red in the ruby.
Similarly, an emerald appears green due to the absorption of red and violet wavelengths and transmission of green. Both rubies and emeralds are coloured by chromium impurity but due to the differences in the strength of ligand fields they exhibit different colours. Ruby has a stronger ligand field; hence absorption is mainly in the green/yellow region and resultant colour is red. In emerald, with weaker ligand field, absorption is mainly in the red/ yellow and violet region and resultant colour is green. In some cases, electrons from the excited state fall back to its normal state or ground state resulting in emission of certain wavelengths. This is known as ‘fluorescence’.
Theories of Gemstone Colour Generation
- Crystal Field Theory
- Molecular Orbital Theory
- Band Gap Theory
- Physical Optics Colour and Optical Effects in Gemstones
- Colour Change Effect – Alexandrite Effect Gemstones
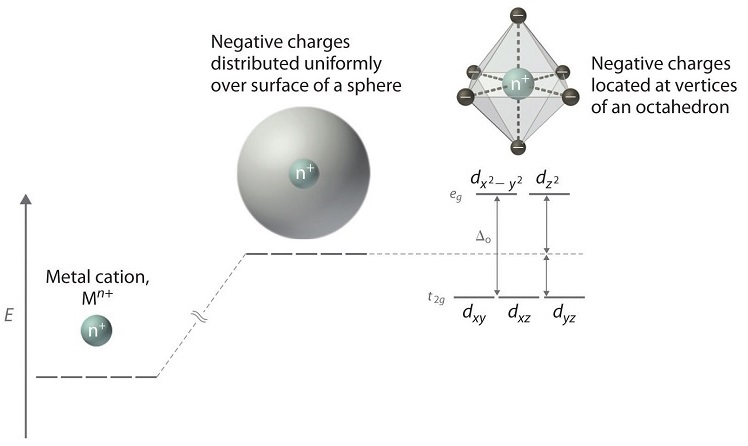
Crystal Field Theory
Unpaired electrons may cause colour in the gemstones. This section describes idiochromatic and allochromatic colouration and colour centres. The transition metal ions may be present in a gemstone as an integral part of its composition (idiochromatic) or as an impurity (allochromatic).
Idiochromatic
These are minerals in which the colour causing transition elements are present as an integral part of the chemical composition, If these minerals are crushed, the powder will also be coloured.
| Gemstone | Chemical Formula | Chromophore | Colour |
|---|---|---|---|
| Almandine Garnet | Fe3Al2(SiO4)3 | Iron (Fe) | Purple, Red |
| Azurite | Cu3(CO3)2(OH)2 | Copper (Cu) | Blue |
| Hematite | Fe2O3 | Iron (Fe) | Red to Brown |
| Malachite | Cu3(CO3)2(OH)2 | Copper (Cu) | Green |
| Peridot | (MgFe)2SiO4 | Iron (Fe) | Green |
| Rhodochrosite | MnCO3 | Manganese (Mn) | Red to Pink |
| Rhodonite | MnSiO3 | Manganese (Mn) | Red to Pink |
| Spessartite Garnet | Mn3Al2Si3O12 | Manganese (Mn) | Orange |
| Turquoise | CuAl6(PO4)(OH)8 .4H2O | Copper (Cu) | Blue |
| Uvarovite garnet | Ca3Cr2Si3O12 | Chromium (Cr) | Green |
Allochromatic
These minerals, in their pure form, are colourless. The colour is caused by small amount of colour causing impurities which are not an essential ingredient of the chemical composition. Example: chromium produces red colour in ruby and green in emerald; absence of chromium impurity will lead to colourless variety. Most of these minerals, when crushed, will produce a colourless powder.
| Gemstone | Chemical Formula | Chromophore | Colour |
|---|---|---|---|
| Alexandrite | BeAl2O4 | Chromium (Cr) | Green to Red colour change |
| Aquamarine | Be3Al2Si6O18 | Iron (Fe) | Blue/Green |
| Blue spinel (synthetic) | MgAl2O4 | Cobalt (Co) | Blue |
| Chrysoberyl | BeAl2O4 | Iron (Fe) | Yellow. Brown |
| Chrysoprase | SiO2 | Nickel (Ni) | Green |
| Citrine quartz | SiO2 | Iron (Fe) | Yellow |
| Emerald | Be3Al2Si6O18 | Chromium (Cr) / Vanadium (V) | Green |
| Grossular Garnet (Tsavorite) | Ca3Al2(SiO4)3 | Chromium (Cr) / Vanadium (V) | Green |
| Jadeite | NaAlSi2O6 | Iron (Fe) | Green/Yellow/Brown |
| Morganite | Be3Al2Si6O18 | Manganese (Mn) | Pink |
| Orthoclase feldspar | KAlSi3O8 | Iron (Fe) | Green to Blue |
| Red beryl | Be3Al2Si6O18 | Manganese (Mn) | Red |
| Red spinel | MgAl2O4 | Chromium (Cr) | Red |
| Ruby | Al2O3 | Chromium (Cr) | Red |
| Spodumene (Kunzite) | LiAlSi2O6 | Manganese (Mn) | Violet/Pink |
| Topaz | Al2SiO4(F,OH)2 | Chromium (Cr) | Red |
| Tourmaline | (Na,Ca)(Li,Mg,Al)3Al6B3Si6O27(OH,F) | Chromium (Cr) Manganese (Mn) Iron (Fe) | Green Red/Pink Green/Blue |
Colour Centres
The colour of gemstones like amethyst, smoky quartz, blue topaz etc. is derived from colour centres. This is due to the defects in the crystal lattice which give rise to defect holes and colour centres. If an electron is missing from its normal position in the crystal structure, it is called a “defect hole” or a “hole colour centre” resulting in absorption of several wavelengths and hence the colour, if an extra electron is present, it is called electron colour centre. Some of the colour centres are stable and lose their colour only on heating, while others are unstable and may fade when exposed to light.
A common example is of Quartz which is silicon dioxide (SiO2) containing silica ions Si4+.Usually such crystals are colourless, most of the natural quartz crystals have Al3+ impurity replacing Si4. If such stone is irradiated (naturally or artificially), one of the paired electrons can be ejected from the original position on an oxygen ion adjacent to the aluminium site. This would leave an unpaired electron, creating a defect hole, which absorbs certain wavelengths of light, thereby producing the smoky colour. The colour produced in this manner is reversible, i.e. if smoky quartz is heated, the electron will come back to its original position and the quartz will become colourless. This smoky colour lightens when the quartz is heated, becoming yellowish-green (lemon quartz) and then colourless at about 4000C. Heating will destroy the defect hole and all the electrons will move to their original positions to make pairs. If Fe3+ (iron) impurity is present in quartz, pale yellow colour is produced due to ligand field. When this quartz is irradiated, amethyst colour is produced. Heating such quartz (amethyst) produces the yellow colour of Citrine.
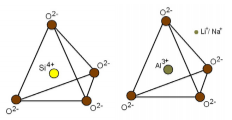
However, the property of change of colour on irradiation and reverting to original colour on heating is not necessarily an evidence of colour centre. Many gemstones show this property due to irradiation induced change in the valence states of the Chromophores. For example, in aquamarine, Fe2+ produces blue colour that is stable to heat while Fe3+ produces a yellow colouration, when both blue and yellow components are present. it results in green colour. On heating, Fe3+ converts to Fe2+ and thus the yellow component changes to colourless while blue component of aquamarine remains unchanged; this can be reversed by irradiation, these changes do not involve colour centres. Unstable defect holes and colour centres can also be destroyed on exposure to light, irradiated yellow sapphire is one common example which fades off within hours or days on exposure to light. Such colour centres or defect holes are termed as ‘fading colour centres’, Colour centre that change their only on heating are termed as ‘stable colour centres’, like in case of smoky quartz.
| Gemstone | Colour |
|---|---|
| Stable colour centres | |
| Amethyst | Purple, Violet |
| Smoky quartz | Brown, Grey |
| Diamond (irradiated) | Greene Blue, Yellow, Brown etc. |
| Topaz (irradiated) | Blue |
| Fluorite | Purple |
| Fading colour centres | |
| Maxixe beryl | Blue to Green |
| Sapphire (irradiated) | Yellow |
| Spodumene (irradiated kunzite) | Green |
Molecular Orbital Theory
Charge Transfer
When an electron is transferred from one transition element to another, it causes absorption of light, and the process is called inter-valence Charge transfer (IVCT). intervalence charge transfer can result in intense colours, as in the case of blue sapphire, The blue colour in sapphire is produced by charge transfer between Fe2+ (iron) and Ti4+ (titanium) ions present at the nearby aluminium (Al3+) sites. One electron from Fe2+ hops to Ti4+, making both trivalent, i.e. Fe3+ and Ti3+; this can be expressed as:
Fe2+ + Ti4+ → Fe3+ + Ti3+
In the process, a certain amount of energy is required, and therefore some wavelengths of light are absorbed, In the above case. most of the wavelengths are absorbed except blue and violet, and as a result, the sapphire appears blue or violet-blue. A deep blue colour results from just a few hundredths of a percent of the required impurities, i.e. titanium (Ti).
The charge transfer may take place between two metallic ions, non-metal to metal, or even two non-metals.
| Gemstone | Colour | Elements Involved | Type of Charge Transfer |
| Iolite | Blue | Fe2+ + Fe3+ | Metal to Metal (same metal) |
| Kyanite | Blue-Green | Fe2+ + Ti4+ | Metal to Metal (different metal) |
| Lapis lazuli | Blue | S3+ | Anion-Anion |
| Sapphire | Blue | Fe2+ + Ti4+ | Metal to Metal (different metal) |
| Tourmaline | Blue and Green | Fe2+ + Fe3+ | Metal to Metal (Same metal) |
Band Gap Theory
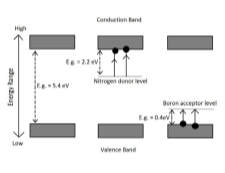
The term “band gap” refers to the energy difference between the top of valence band (the highest range of energy where electrons are normally present) and the bottom of the conduction band (the range of energy where the electrons get excited and accelerate), Electrons jump from one band to the other by absorbing some energy and thereby produce colour.
This mainly happens in metals or semi-conductors. Only a few stones are coloured by this mechanism like diamond and galena. In diamond the presence of nitrogen or boron gives colour and forms different types of band gaps. The colour of such a gem material depends on the Size of the band gap. If the band gap is large, then no wavelength of light in the visible spectrum is absorbed (everything is transmitted), hence a stone will appear colourless. However, if band gap lies within the visible range, some of the wavelengths will be absorbed While some transmitted, resulting in colour- In case of diamond, nitrogen acts as a donor and absorbs violet or blue wavelengths and hence appears yellow, while boron acts as an acceptor and absorbs red/yellow wavelengths and results in blue colour.
Physical Optics-Colour and Optical Effects
The interaction of light with the physical structure of stone may give rise to various optical effects like dispersion, scattering, diffraction, thin film interference, etc.
Scattering of light
The blue colour of sky is the result of scattering of light by fine particles (dust or gases) in the atmosphere. Shorter wavelengths of light (violet and blue) are scattered more than the longer wavelengths (red/yellow). As the Shorter wavelength (blue) is scattered, the sky appears blue during the day. During sunrise and sunset most of the light strikes tangential to the earth’s surface. Therefore, the path of light through the atmosphere is long and most of the blue and green light is scattered removing blue light from the direct path of the observer. The remaining light is mostly of longer wavelength and thus the sky and the clouds appear red. This also happens during dust storms. As more dust particles get suspended in the atmosphere, more rays get scattered and as a result the sky appears yellow or orange or red, if there was no atmosphere on earth, the sky would be black even during the day, (like on moon or some planets without atmosphere) as there would be no particles to scatter light. In gemstones. the best example of scattering is that of moonstone feldspar. The blue sheen or “adularescence” seen on the surface of the moonstone is the result of scattering of light from minute particles of albite in the layered structure. This scattering, in conjunction with interference from the layers, gives rise to blue sheen.

Reflection Of Light
Cat’s eye effect (chatoyancy) and Asterism: If the gemstone has a series of microscopic needle-like parallel inclusions and the gemstone is cut as cabochon, it shows a single bright ray of light running across the stone. This ray appears to move with the movement of the source of light or the stone itself. The stone is cut such that the needles are parallel to the base of the cabochon. The chatoyancy effect is due to the reflection of light from the needle like inclusions. The gemstones known to exhibit chatoyancy are quartz, tourmaline, apatite, diopside, chrysoberyl etc.
If the fine needle like inclusions are parallel to more than one crystal face, a star effect or asterism is produced when the gemstone is cut as cabochon with the base parallel to the plane containing the needles, Asterism may be seen in ruby, sapphire, rose quartz, garnet etc.

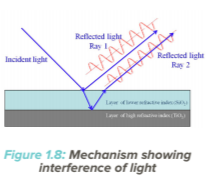
Interference
The iridescent colours exhibited by a thin film of oil floating on water are caused by interference of light. Rays of light which strike the surface of a gem are partially reflected back and partially refracted into the gem. If a gem has a layered structure, the refracted rays will again reflect back from the layers beneath the top surface. Since, some rays have travelled a longer distance, they may be out of phase with the other ones, resulting in interference between the reflected rays from the surface and from within the stone. Such interference will give rise to colours as seen in fire agate. along cleavage planes, or in some coated gemstones, A common gem material which exhibits interference is labradorite feldspar. commonly referred to as “rainbow moonstone”. It is known for its bright spectral coloured flashes on the surface. Labradorite is composed of parallel layers of many twin lamellae of varying and alternating composition giving rise to layers of alternating optical densities, Iridescent colours are produced by interference in these layers if the arrangement is regular, uniform and fine enough to produce interference
Diffraction
Diffraction is the phenomenon of bending of light around small objects of tiny openings, The most Striking example is that of a compact disc or DVD which has fine grooves. The closely packed groves act as a diffraction grating to produce the bright spectral rainbow colours. Opal is a prime example of a gemstone which exhibits brilliant “play of colour” due to diffraction. Opal consists of a three-dimensional close packed structure of spheres of silica. These spheres form a three-dimensional grating and cause diffraction of light which results in “play of colour”.

Colour Change Effect – Alexandrite Effect
The colour-change effect, commonly known as “alexandrite effect” refers to the change in the body colour of the stone when observed in different types of light sources. typically, fluorescent daylight and incandescent light. In case of alexandrite, colour typically changes from green in daylight to red in incandescent light.
Chromium impurity produces the red colour in ruby and the green in emerald due to the differences in the strength of their ligand-fields. In alexandrite, the ligand field is intermediate between ruby and emerald, which causes the colour change effect. The colour change effect is produced when the transmission band in the red part of spectrum and the blue-green part are balanced. Thus, when observed under daylight (rich in blue wavelengths), the stone appears blue-green, while under incandescent light (rich in red wavelengths), the stone appears purple-red or brownish-red.
Some of the other stones that show colour change effect include Zultanite (colour-change diaspore), synthetic sapphire with high percentage of vanadium and glasses like Alexite where the colour change effect is due to rare earth elements.

References
- CIE 1931 color space, 20 Feb. 24, https://en.wikipedia.org/wiki/CIE_1931_color_space
- Color wheel, 20 Feb. 24, https://en.wikipedia.org/wiki/Color_wheel
- Electromagnetic spectrum, 20 Feb. 24, https://en.wikipedia.org/wiki/Electromagnetic_spectrum
- Kurt Nassau, The Physics and Chemistry of Color 2nd ed., 2001
- Joel E. Arem, Color Encyclopedia of Gemstones, 1987
- Crystal Field Theory, 21 Feb. 24, https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Crystal_Field_Theory/Crystal_Field_Theory
- Molecular Orbital Theory, 21 Feb. 24, https://chem.libretexts.org/Courses/City_College_of_San_Francisco/Chemistry_101A/Topic_F%3A_Molecular_Structure/10%3A_Orbitals_and_Bonding_Theories/10.4%3A_Molecular_Orbital_Theory